About Us
Our History
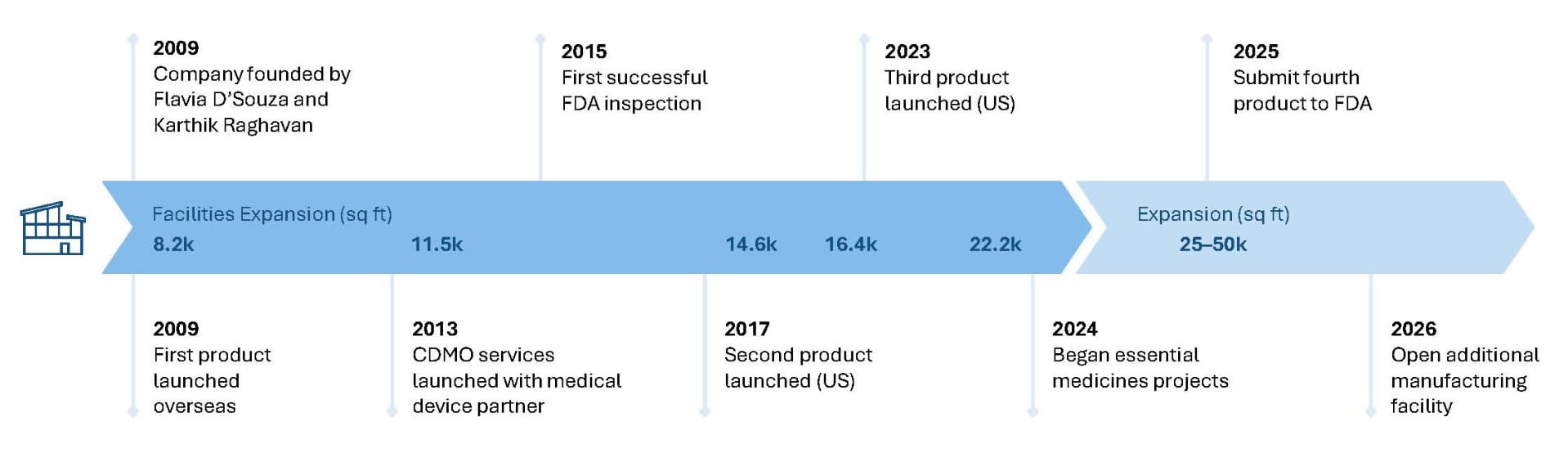
Sentio BioSciences LLC was founded in 2008 by Karthik Raghavan and Flavia D’Souza, with setup of the Active Pharmaceutical Ingredient (API) commercial manufacturing facility in Maryland Heights (St. Louis County), Missouri.
Our Team
Sentio has a diverse and performance driven team supporting all cGMP and business areas.
Our Accomplishments
- FDA Approved New and Generic Products on the Market
- Continued Regulatory Compliance with FDA, OSHA, DEA, Missouri DNR, Missouri MSD, and EPA


- Research and Development completed in the US
- cCMP Manufacturing completed in the US with numerous successful FDA audits
- Re-shoring Manufacturing of Complex APIs to the US
- Analytical method development, validation and testing of complex non-compendia methods approved by the FDA.
- Vertically Integrated Manufacturing (Manufacture Raw material to Finished Drug Products)
- Flexible platform technology for multiple product manufacturing
- Advanced manufacturing technologies including process controls and in-process analytics, flow and continuous manufacturing, and automation.
Mission & Values
Our Mission
Sentio BioSciences LLC is committed to providing the highest quality product to our customers, while always maintaining the most compliant and safe business model. Commitment to fulfill customer expectations is our primary goal. We use our resources and competencies to deliver the highest quality and exceptional service. We promote continuous quality improvement in every facet of our operation.
Our Values
INNOVATIVE
Seek novel ideas, approaches, and customer-centric collaborations
RESPONSIBLE
Maintain highest levels of integrity, transparency, safety and fiscal accountability
BOLD
Be courageous in goals, approach and timely decisions
AGILE
Be urgent, time-bound, and continuously improving
CELEBRATE
Recognize achievements big and small, support performance and growth
DIVERSE
Win with performance-driven diverse teams, expertise, and experiences
Our Key Alliances
Career At Sentio
Why Work at Sentio?
- Work with Diverse and Collaborative Teams
- Opportunity to Learn all aspects of Pharmaceutical Industry
- Opportunity to Work on Small and Large Complex Projects
- Pride in bringing complex and essential medicines to market
- Making a difference in Human and Animal Health through our Medications
- Opportunities for Professional Growth and Skills Development
News
- 25 Jul 2025 Sentio BioSciences Earns Prestigious SHARP Award for Excellence in Workplace Safety.
Read more … - 9 June 2025 Sentio BioSciences to expand in Maryland Heights, investing more than $10.6 million and creating 58 new jobs.
Read more … - 12 December 2024 API Innovation Center Awards Contract to Sentio BioSciences to Advance Development of Two Critical Active Pharmaceutical Ingredients – API Innovation Center.
Read more … - October 2024 Sentio is registered as DEA Schedule II to V Researcher.
Read more … - 18 September 2024 Flavia D’Souza, Co-Founder and Head of Quality was elected on the BioMap Consortium Executive Steering Committee.
Read more … - 20 July 2023 Fire Awards 2023: Sentio Biosciences brings new drug to market, works on another while expanding operations
Read more … - 10 May 2023 The API Innovation Center (APIIC) to Solidify U.S. Active Pharmaceutical Ingredient (API) Manufacturing Base Through Strategic Partnerships
Read more … - 02 January 2023 St. Louis area firms win federal approval for new horse drug
Read more … - 20 December 2022 FDA Approves First Injectable Pentosan for Osteoarthritis in Horses
Read more … - 30 June 2022 Risk pays off as Indian immigrant builds a drug manufacturer in St. Louis
Read more … - 17 February 2017 PRESS RELEASE: FDA Approves Diroban, the First Generic Drug to Treat Heartworm Disease in Dogs
Read more …
Innovation | Collaboration | Commercialization